mixture of H2 and O2 gases or water
수소 기체와 산소 기체는
물을 형성하기 위하여 격렬하게 반응한다.
어느 것이 더 낮은 에너지인가?
수소와 산소의 혼합물 혹은 물 중 선택하고
그 이유를 설명하라.
Hydrogen gas and oxygen gas
react violently to form water.
Which is lower in energy:
a mixture of hydrogen and oxygen gases or water?
Explain.
[참고] 발열반응 흡열반응
[ https://ywpop.tistory.com/5242 ]
격렬하게 반응
---> 에너지가 방출되는 발열 반응이다.
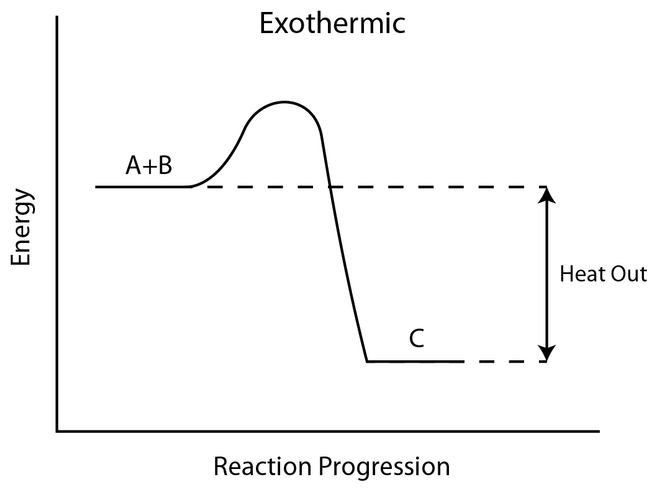
[ 그림 출처 Wikimedia ] Energy level diagram of an exothermic reaction.
발열 반응
: 반응물의 에너지 준위 > 생성물의 에너지 준위
답: 생성물인 물(water)
This reaction is violent,
meaning a significant amount of energy is released.
This means that the products of the reaction (water)
have a lower potential energy than the reactants.
In an exothermic reaction like this,
the products are lower in potential energy
than the reactants.
So, the final answer is:
The mixture of hydrogen and oxygen gases
is higher in energy than water.
This is because the reaction is exothermic,
meaning energy is released during the reaction,
lowering the potential energy of the products (water).
This can be represented in an energy-level diagram,
where the reactants are at a higher energy level
than the products.
[키워드] 발열반응 기준

YOU MIGHT LIKE
모두 보기댓글 쓰기